At a pH of 6, the concentration of H+ ions is 1 x 10^(-6) M and the concentration of OH- ions is 1 x 10^(-8) M. The ratio of H+ ions to OH- ions at a pH 6 is 100.
The concentration of H+ ions at a pH 6 is calculated using the formula: pH = -log[H+].
Rearranging the formula, we get [H+] = 10^(-pH).
Substitute the pH value of 6 into the equation: [H+] = 10^(-6) = 1 x 10^(-6) M.
Therefore, the concentration of H+ ions at a pH 6 is 1 x 10^(-6) M.
To find the concentration of OH- ions at a pH 6, we use the equation: [H+] x [OH-] = 1
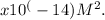
Since we know the concentration of H+ ions is 1 x 10^(-6) M, we can substitute this value into the equation: (1 x 10^(-6)) x [OH-] = 1 x 10^(-14).
Divide both sides of the equation by 1 x 10^(-6) to solve for [OH-]: [OH-] = 1 x 10^(-14) / 1 x 10^(-6) = 1 x 10^(-8) M.
Therefore, the concentration of OH- ions at a pH 6 is 1 x 10^(-8) M.
The ratio of H+ ions to OH- ions at a pH 6 can be calculated by dividing the concentration of H+ ions by the concentration of OH- ions: (1 x 10^(-6)) / (1 x 10^(-8)) = 100.
The ratio of H+ ions to OH- ions at a pH 6 is therefore 100.