Answer:
0.47 M
Step-by-step explanation:
The concentration of the solution can be calculated using the following equation:
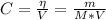
Where:
V: is the volume of the solution = 68.6x10⁻² L
η: is the moles of cobalt (II) sulfate
m: is the mass of cobalt (II) sulfate = 89.94 g
M: is the molar mass of cobalt (II) sulfate = 281.103 g/mol
The concentration of cobalt (II) sulfate is:
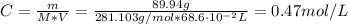
We used the molar mass of the cobalt (II) sulfate heptahydrate (281.103 g/mol) since it is one of the most common salts of cobalt.
Therefore, the concentration of a solution of cobalt (II) sulfate is 0.47 M (assuming that the cobalt (II) sulfate is heptahydrate).
I hope it helps you!