Answer:
![[Ag^+]=1.3x10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/l6yukz0qzkwz51u5zvci6jzrgk5lrmqqv0.png)
Step-by-step explanation:
Hello,
In this case, given the solubility product of silver chromate:
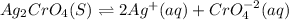
Now, the law of mass action excluding silver chromate as it is solid, turns out:
![Ksp=[Ag]^2[CrO_4^(-2)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/kaeqt0t7q348x7krqzyc17mnmh6dv2li6m.png)
Thus, given the change
 due to the dissolution of silver chromate, we obtain:
due to the dissolution of silver chromate, we obtain:
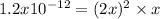
Hence, we solve for
 :
:
![x=\sqrt[3]{(1.2x10^(-12))/(2^2) } = 6.7x10^(-5)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/8ualzz9vccnpfxqc2bag6emj97d243rgjy.png)
Thus, the concentration of silver ions will be:
![[Ag^+]=2x=2*6.7x10^(-5)M=1.3x10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/p2qzwkxeitffke0vy3ii6n563mjhtbd1lr.png)
Best regards.