Answer: dilute
Step-by-step explanation:
A concentrated solution which is used to prepare solutions of lower concentrations by diluting it with addition of water.
A dilute solution is one which contains lower concentration.
Using Molarity equation:
 =concentration of stock solution = 0.150 mol/L
=concentration of stock solution = 0.150 mol/L
 = volume of stock solution = 10.0 ml
= volume of stock solution = 10.0 ml
 = concentration of dilute solution = ?
= concentration of dilute solution = ?
 = volume of dilute solution = (10.0+90.0) ml = 100.0 ml
= volume of dilute solution = (10.0+90.0) ml = 100.0 ml
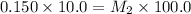
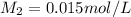
As the concentration is less than the original concentration, the solution is termed as dilute.