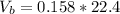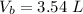Complete Question
What volume of butane can be produced from the reaction of 13.45 g of carbon and 17.65 L of hydrogen gas at STP ?
Answer:
The volume of butane produced is
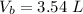
Step-by-step explanation:
From the question we are told that
The mass of carbon is
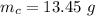
The volume of hydrogen is
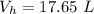
The number of moles of carbon is mathematically evaluated as
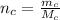
Where
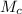 is the molar mass of carbon which is a constant with value
is the molar mass of carbon which is a constant with value
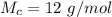
So
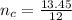
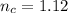
Now since the volume of the hydrogen is measured at standard temperature and pressure
Hence the number of moles of hydrogen is
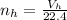
Where 22.4 is a constant
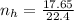
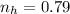
Comparing
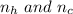 we see that hydrogen is the limiting reactant
we see that hydrogen is the limiting reactant
because
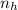 is greater than
is greater than
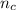
The chemical equation for this reaction is

looking at chemical equation we see that
5 moles of hydrogen gas reacts with 4 moles of carbon to produce 1 mole of butane
This implies that
0.79 moles react with 1.12 moles of carbon to produce x moles of butane
Therefore
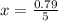
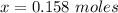
Now since this reaction is carried out at standard temperature and pressure the volume of butane produced will be