Answer:
2.15 moles of chlorine gas
Step-by-step explanation:
given data
volume = 29 L
temperature = 300 K
pressure = 1.8 atm
solution
we will apply here ideal gas equation formula
PV = nRT .......................1
and here R is R = 0.082 l atm/ K mol
so n will be here as express as
n =
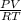
n =
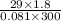
n = 2.15 moles
so 2.15 moles of chlorine gas