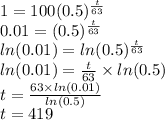Answer:
Half-life = 63 years
Mystery element is Titanium-44
There will be 1 gram left after 419 years have passed
Explanation:
In general the equation describing half-life is:
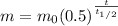
Where m is the final mass, m0 is the starting mass, t is the total elapsed time, and t(1/2) is the length of the half life. t / t(1/2) is actually the number of half-lives that have passed.
Anyways - all your questions can be answered using the above equation.
What is the half-life?
I subbed in the values we know from the question. In solving this I used the natural logarithm (but a log of any base will do - the goal is to bring down the exponent):
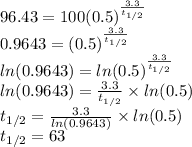
What is the mystery element?
Titanium-44, because it's half-life in the table is closest to the calculated half-life.
When will there be 1 gram left?
Again use the equation. This time the total time, t, is unknown. We can use the previously calculated half-life: