Answer:
A 3.1 M solution contains 3.1 moles of solute dissolved in 1 liter of solution.
The M in 3.1 M refers to molarity.
The concentration of a 3.1 M solution may also be expressed as
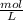 and would be expressed in
and would be expressed in
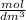
Step-by-step explanation:
The molarity reflects the concentration of a solution indicating the amount of moles of solute that appear dissolved in each liter of the solution.
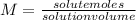
Although various units can be used, it is usual to use
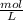 , equivalent to
, equivalent to
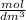 . This unit is usually expressed as a molar, with an M as a symbol.
. This unit is usually expressed as a molar, with an M as a symbol.