Answer:
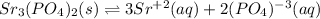
![Ksp=[Sr^(+2)]^3[(PO_4)^(-3)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/r039lce4ecnyc5xnfquwtl5gt5e62yhrky.png)
Step-by-step explanation:
Hello,
In this case, for strontium phosphate, we find an ionic equation for its dissociation as shown below:
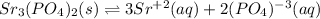
Next, the solubility product is found by applying the law of mass action, considering that the solid salt is not considered but just the aqueous species due to heterogeneous equilibrium:
![Ksp=[Sr^(+2)]^3[(PO_4)^(-3)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/r039lce4ecnyc5xnfquwtl5gt5e62yhrky.png)
Best regards.