Answer:
MCl₂
Step-by-step explanation:
The formula for boiling point elevation can be used to find x. The "complete dissociation" means there will be an ion of M and x ions of Cl in the solution. The number of moles of solute will be 30.2 grams divided by the molecular weight of MClx, where x is the variable we're trying to find.
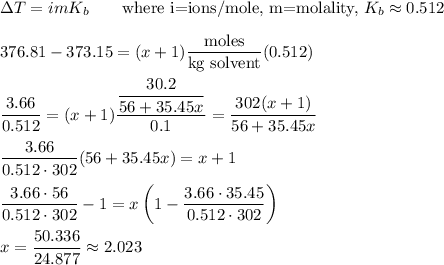
Then the formula for the salt is MCl₂.