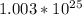Answer:
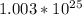
Step-by-step explanation:
We'll be using Avogadro's number which defines the amount of atoms in a mole.
To find the mole amount, we use the atomic mass to find the molar mass because the atomic mass represents how many grams are in a single mole of any element
2 Hydrogen atoms have an atomic mass of 2.016 g
1 Oxygen atom has an atomic mass of 15.999 g
Adding these two numbers gives us H2O's molar mass, which is 18.015 g/mol (grams per mole).
Now we take the supplied mass of 300g and divide it by the mm (molar mass) to find the amount of moles we have. 300/18.015 = 16.653
We have 16.653 moles in 300g of H2O.
Now we have to multiply 16.653 by Avogadro's number,
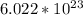
This gives us: