Answer:
![p[H+] = 10.042](https://img.qammunity.org/2021/formulas/chemistry/high-school/a3npi018uddrpqnf4x35i2ryyqir582bb6.png)
Step-by-step explanation:
As we know that
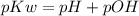 ......eq (1)
......eq (1)
we will calculate the pH of OH- and then we will calculate the pH of H+
So p[OH-]
![= - log [1.10 * 10^(-4)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/bfwft3ljonypob7nei6tizdxwn3h9zbb12.png)
Solving the right side of the equation, we get
p[OH-]
![= - [-3.958]\\= 3.958](https://img.qammunity.org/2021/formulas/chemistry/high-school/8swf3boc21fq8quqibdw8tvmun5oqg2jrm.png)
Now we know that
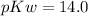
Substituting the value of pOH in the above equation, we get -
![14.0 = p[H+] + 3.958\\p[H+] = 14 - 3.958\\p[H+] = 10.042](https://img.qammunity.org/2021/formulas/chemistry/high-school/rh4hefkoufx7z8katrs28ptc6xihmwlw3w.png)