Answer:
atomic percentage = 143 %
Step-by-step explanation:
Let x be the number of tin atoms and there are 4 atoms / cell in the FCC structure , then 4 -x be the number of copper atoms . Therefore, the value of x can be determined by using the density equation as shown below:

where;
the lattice parameter is given as : 4.7589 × 10⁻⁸ cm
The atomic mass of tin is 118.69 g/mol
The atomic mass of copper is 63.54 g/mol
The density is 8.772 g/cm³
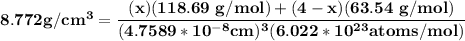
569.32 = 118.69x + 254.16-63.54x
569.32 - 254.16 = 118.69x - 63.54 x
315.16 = 55.15x
x = 315.16/55.15
x = 5.72 atoms/cell
As there are four atoms per cell in FCC structure for the metal, thus, the atomic percentage of the tin is calculated as follows :
atomic % =
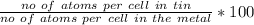
atomic % =
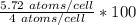
atomic % = 143 %