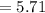Answer:
Volume will the gas occupy at
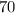 kPa
kPa
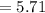
Step-by-step explanation:
As per the gas law
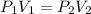
Here
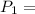 initial pressure of the gas
initial pressure of the gas
 = final pressure of the gas
= final pressure of the gas
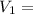 Initial volume of the gas
Initial volume of the gas
 = the final volume of the gas
= the final volume of the gas
here,
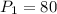 kPa
kPa
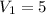 mL
mL
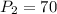
Substituting the given values in above equation, we get -
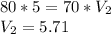
Volume will the gas occupy at
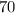 kPa
kPa