Answer: Superscript 32 subscript 15 upper P right arrow superscript 32 subscript 16 upper S plus superscript 0 subscript negative 1 e.
Step-by-step explanation:
Beta Decay : It is a type of decay process, in which a proton gets converted to neutron and an electron. This is also known as -decay. In this the mass number remains same but the atomic number is increased by 1.
The general representation of beta minus emission is :
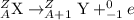
The representation of beta decay of phosphorous- 32 :
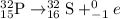
Superscript 32 subscript 15 upper P right arrow superscript 32 subscript 16 upper S plus superscript 0 subscript negative 1 e.