Answer:
see below for explanation
Step-by-step explanation:
1. you need to set up and balance the equation
NaOH + HC2H3O2 --> NaC2H3O2 + H2O
2. Write the value they gave you underneath the equation to figure out what we are solving for, which is the molarity of NaOH
NaOH (Base) + HC2H3O2 (Acid)--> NaC2H3O2 + H2O
15.0 ml | 7.5ml
? M | 0.02M
3. Use the MaVa=MbVb ·
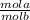
- This formula is saying the M of an acid times the volume of the acid = the M of the base times the volume of the base times the mol of the acid/ mol of the base ratio. ***You should always compute the volume in Liters.
- Convert your values (x is the molarity of the base, the unknown value) Our acid values are first because that is how the equation is set up
0.0075 · 0.02 = 0.0150 · x · 1/1
4. Simplify
0.00015 = 0.0150x
5. Solve
Molarity of the base = 0.01