Answer:
2.56
Step-by-step explanation:
What is the pH of a solution that contains 5.23g of hydrochloric acid dissolved in 52.32L of water?
Step 1: Calculate the moles of hydrochloric acid
The molar mass of hydrochloric acid is 36.46 g/mol. The moles corresponding to 5.23 g are:
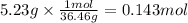
Step 2: Calculate the molar concentration of hydrochloric acid
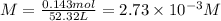
Step 3: Calculate the molar concentration of H⁺
Hydrochloric acid is a strong monoprotic acid that dissociates according to the following expression.
HCl(aq) ⇒ H⁺(aq) + Cl⁻(aq)
Then, [H⁺] = 2.73 × 10⁻³ M
Step 4: Calculate the pH of the solution
We use the following expression.
pH = -log [H⁺]
pH = -log 2.73 × 10⁻³
pH = 2.56