Answer:
Option B,
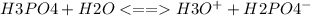
Step-by-step explanation:
In this reaction, a weak acid is reacting with water. Thus, water is this case will act as a proton acceptor or a base as well as an acid. Hence water will be amphiprotic for this chemical process and can donate as well accept as a proton. Now when weak acid such as phosphoric acid loses a hydrogen ion it forms a weak conjugate base ie. H2PO4^-. Water being a weak base shall accept the proton and forms hydronium ion i.e H3O^+
The dihydrogen phosphate ion reacts with water:
H2PO4^- + H2O <----> HPO4^2- + H3O^+
After some time a proton is again transferred to the H2O molecule to produce phosphate ion
HPO4^2- + H2O <----> H3O^+ + PO4^3-