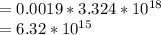Answer:

Step-by-step explanation:
The molar concentration is defined as the number of moles of a substance in one unit volume of a solution
Here
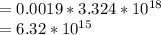
Molar concentration
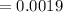 M
M
The dimensions of bacterial cell are as follows
Diameter
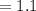 μm
μm
and length of the bacterial cell
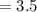 μm
μm
The volume of the solution is equal to product of area and the length
Thus, volume
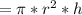
Substituting the given values in above equation, we get -
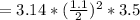
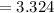 μm^3
μm^3
Number of molecules