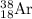Answer: There are 20 neutrons in the isotope
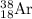
Step-by-step explanation:
In isotope
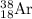 , 18 represents atomic number and 38 represents mass number.
, 18 represents atomic number and 38 represents mass number.
Atomic number : It is defined as the number of electrons or number of protons present in a neutral atom.
Thus, number of protons = atomic number = 18
Mass number is the number of the entities present in the nucleus which is the equal to the sum of the number of protons and electrons.
Mass number = Number of protons + Number of neutrons = 38
Thus : 38 = 18 + Number of neutrons
Number of neutrons = 20
Thus there are 20 neutrons in the isotope