Answer:
d . Sc2O5
Step-by-step explanation:
Hello,
In this case, when forming oxides from a metal and oxygen, for us to find out each element's subscript, we must exchange them as shown below, considering +5 for scandium:
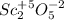
For that reason, the answer is d . Sc2O5
Best regards.