Answer:
Step-by-step explanation:
We shall apply gas law equation
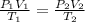
P₁ = 221 X 10³
V₁ = 255 L
T₁ = 273 + 125
= 398 K
P₂ = 1.01 x 10⁵ ( Standard pressure )
V₂ = ?
T₂= 273 K ( standard temperature is 0 degree )
Putting the values above in the gas equation
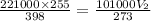
V₂ = 382.73 L .