Answer:
The average binding energy per nucleon of
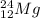 is 8.00 MeV/nucleon
is 8.00 MeV/nucleon
Step-by-step explanation:
Here we have the molar mass of
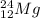 is 23.985042 amu
is 23.985042 amu
The mass of a proton
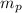 = 1.007276 amu
= 1.007276 amu
The mass of a neutron
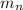 = 1.008665 amu
= 1.008665 amu
From the notation, 1 Mg = has 12 protons and 12 neutrons
Therefore, we find the binding energy as follows;
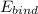 = 12 × (1.007276) amu + 12 × (1.008665) amu - 23.985042 amu
= 12 × (1.007276) amu + 12 × (1.008665) amu - 23.985042 amu
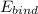 = 24.191292 amu - 23.985042 amu = 0.20625 amu
= 24.191292 amu - 23.985042 amu = 0.20625 amu
1 amu = 931 MeV
Therefore, 0.20625 amu = 0.20625 amu × 931 MeV = 192.01875 MeV
The average binding energy per nucleon in
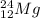 which has 24 nucleons, is thus;
which has 24 nucleons, is thus;
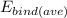 = 192.01875 MeV ÷ 24 = 8.00078 MeV/nucleon ≈ 8.00 MeV/nucleon.
= 192.01875 MeV ÷ 24 = 8.00078 MeV/nucleon ≈ 8.00 MeV/nucleon.