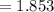Answer:
pH
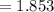
Step-by-step explanation:
For every mole of hydrochloric acid, one mole of hydronium ion is required. Thus, in order to neutralize 0.014 moles of HCL, 0.014 moles of hydronium is required.
![[H_3O^+] = [HCl] = 0.014](https://img.qammunity.org/2021/formulas/chemistry/high-school/6jmmstl1ueubwr1cys9savzbpvrsvc3isq.png)
pH
![= -log [H^+] = -log [H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/1rpe0p158wvw0wzkugtge0e2p2sck8if31.png)
Substituting the available values in above equation, we can say that the pH of the solution is equal to
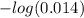
pH
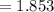
pH of a
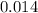 M HCL solution
M HCL solution