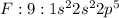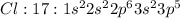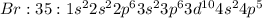Answer: A. need the same number of electrons to fill their valence shells
B. have the same number of valence electrons
Step-by-step explanation:
Elements are distributed in groups and periods in a periodic table.
Elements that belong to same groups will show similar chemical properties because they have same number of valence electrons.
Example: Flourine, chlorine and bromine are elements which belong to Group 17. They have 9, 17 and 35 electrons respectively and contain 7 valence electrons each and need one electron to complete their octet.
The chemical reactivity of elements is governed by the valence electrons present in the element.