Answer:
694.2915 grams of potassium chlorate is needed to produce 8.50 mol of oxygen
Step-by-step explanation:
It is possible to apply the following rule of three: if by stoichiometry 3 moles of O₂ are produced by 2 moles of KCIO₃, when reacting 8.5 moles of O₂, how many moles of KClO₃ are necessary?
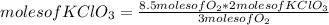
moles of KClO₃= 5.67
To know the amount of mass present in 5.67 moles of the compound, it is necessary to know the molar mass, that is, the amount of mass that a substance contains in one mole. Being:
- K: 39 g/mole
- Cl: 35.45 g/mole
- O: 16 g/mole
The molar mass of the compound is:
KClO₃= 39 g/mole + 35.45 g/mole + 3* (16 g/mole)= 122.45 g/mole
Then you can apply the following rule of three: if in 1 mole of the compound there are 122.45 grams, in 5.67 moles how much mass is there?
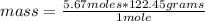
mass= 694.2915 grams
694.2915 grams of potassium chlorate is needed to produce 8.50 mol of oxygen