Answer:
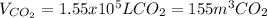
Step-by-step explanation:
Hello,
In this case, given the reaction:
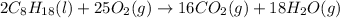
The total consumed gallons are computed by considering 686 miles were driven and the consumption is 21.2 miles per gallon, thus:
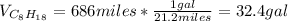
Hence, with the given density, one could compute the consumed grams and consequently moles of gasoline as well as moles that were consumed:
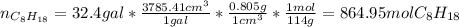
Next, since gasoline (molar mass = 114 is in a 2:16 molar relationship with the yielded carbon dioxide, we compute its produced moles as shown below:
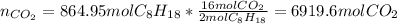
Finally, we could assume the given STP conditions to compute the volume of carbon dioxide, as no more information regarding the space wherein the carbon dioxide is available:
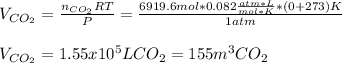
Best regards.