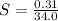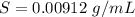Answer:
The solubility is
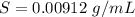
Step-by-step explanation:
From the question we are told that
The original volume of sample is
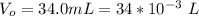
The temperature is
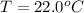
The new volume of sample is
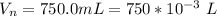
The weight of the crystal is
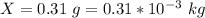
Now looking at the question we see that 34.0 mL of the sample is saturated with 0.31g of the crystal X
Generally the solubility of X in the water sample at
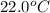 can be mathematically evaluate as
can be mathematically evaluate as