Answer:
Q4. 2H₂ + O₂ ⟶ 2H₂O; 5; 2
Q5. 24; 30; H₂; 0; 5
Step-by-step explanation:
Q4.
The equation for the reaction is
2H₂ + O₂ ⟶ 2H₂O
One O₂ molecule reacts with 2H₂ molecules.
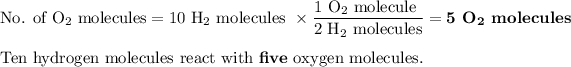
We started with seven oxygen molecules. Five of them reacted, so
Two O₂ molecules did not react.
Q5.
Two water molecules form from two hydrogen molecules.
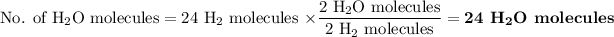 Two water molecules form from one oxygen molecule
Two water molecules form from one oxygen molecule
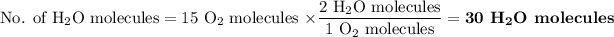 Hydrogen forms fewer water molecules of water, so
Hydrogen forms fewer water molecules of water, so
Hydrogen is the limiting reactant.
The 24 hydrogen molecules will be completely used up.
The number of hydrogen molecules remaining at the end of the reaction is zero.
They have reacted with 12 O₂ molecules.
The number of O₂ molecules remaining is 17 - 12 = 5