Answer:
86.2% of Hg in the ore
Step-by-step explanation:
Percent composition is defined as one hundred times the ratio between mass of an element in a compound and total mass of the compound. Formula is:
 % composition =
% composition =
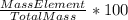
If an ore weights 681g and contains 587g of mercury, percent composition is:
(587g Hg / 681g) × 100 = 86.2% of Hg in the ore