Answer:
0.134 moles
Step-by-step explanation:
In order to solve this, we need to find the molar mass of Chromium, which we can see from the periodic table: it is 51.996 g/mol.
Now, we need to set up an equation to convert grams to moles:
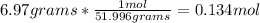
Thus, the answer is 0.134 moles of Chromium.