Answer:
The final temperature is 414.34 degrees Celsius.
Step-by-step explanation:
Gay-Lussac's law gives the relation between pressure and temperature. It states that at constant volume, pressure is directly proportional to the temperature of the gas.
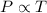
We have,
Initial pressure,
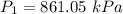
Initial temperature,
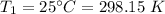
Final pressure,
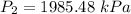
It is required to find new temperature, using Gay-Lussac's law as:
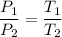
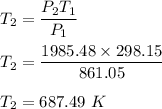
or
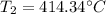
So, the final temperature is 414.34 degrees Celsius.