Answer: pH of an
 solution is 4.34
solution is 4.34
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rjo2yhb5oj9ry1fr4db1ujrazm6fh3vhke.png)
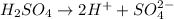
Putting in the values:
![pH=-\log[4.58* 10^(-5)]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/coyep9u7uhg9k402d3oqlq87ip0rq2dh0h.png)
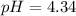
Thus pH of an
 solution if the
solution if the
![[H^+]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/rjqz56ud9q34ms188mnkhp8tjhwy4eib74.png) is
is
![4.58* 10^(-5)]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/5g3y76e6twkumia1tb4y4xyrqn58q66fhy.png) is 4.34
is 4.34