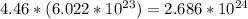Answer:
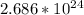
Step-by-step explanation:
Avogadro's number, 10 x 10^{23}, represens the number of atoms in a single mole of any substance.
Since we already of have the mole amount and don't need to calculate that from the grams of the substance, we can simply multiple Avogadro's number by the amount of moles given.