Answer:
0.283 moles of lead (IV) chloride are needed to completely react with 42.0 grams of calcium hydroxide.
Step-by-step explanation:
First of all you must know the amount of mass of calcium hydroxide that reacts by stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction). For that you must know the molar mass of the compound. So, being:
- Ca: 40 g/mole
- O: 16 g/mole
- H: 1 g/mole
the molar mass of calcium hydroxide is:
Ca(OH)₂= 40 g/mole + 2*(16 g/mole + 1 g/mole)= 74 g/mole
If 2 moles of sodium hydroxide react with stoichiometry, then:
Ca(OH)₂=2 moles* 74 g/mole= 148 g
Now you apply the following rule of three: if 148 grams of calcium hydroxide react with stoichiometry with 1 mole of lead (IV) chloride, how many moles of lead (IV) chloride would react with 42 grams of the hydroxide?
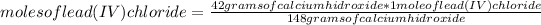
moles of lead (IV) chloride=0.283 moles
0.283 moles of lead (IV) chloride are needed to completely react with 42.0 grams of calcium hydroxide.