Answer:
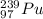
Step-by-step explanation:
The capture of an alpha particle (
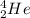 ) by the
) by the
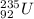 is:
is:
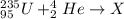
The capture of an alpha particle by the uranium produces an increase in the atomic number of the uranium in four (because of the two protons and two neutrons of the alpha particle) and an increase of the protons number in two.
Therefore, the nuclide is:
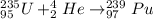
Hence, the correct option is the third option:
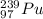
I hope it helps you!