Answer:
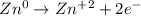
Step-by-step explanation:
Hello,
In this case, the described chemical reaction is:
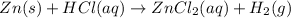
Then, to identify the half reactions we must assign the oxidation state for each element as shown below:
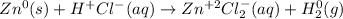
In such a way, we can notice zinc's oxidation state changes from 0 to +2, therefore, it is the oxidizes species for which the half reaction which correctly describes the oxidation that is taking place is:
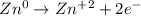
Best regards.