Answer:
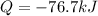
Step-by-step explanation:
Hello,
In this case, for such formation of sulfur hexafluoride, the standard enthalpy of formation is -1220.47 kJ/mol (data extracted from NIST database). Next, we compute the moles in 10.0 grams of sulfur hexafluoride as shown below:
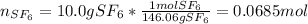
Next, for the given energy, we compute the total heat that is liberated:
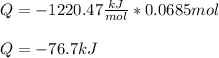
Finally, we conclude such symbol has sense since negative heat is related with liberated heat.
Best regards.