Answer:
D. 91.0%
Step-by-step explanation:
Hello,
In this case, for the given chemical reaction:
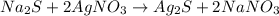
Next, since silver nitrate (molar mass 169.87 g/mol) is in a 2:1 molar ratio with silver sulfide (molar mass 247.8 g/mol), we compute its theoretical yield as shown below:
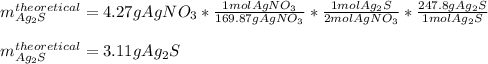
Next, we compute the percent yield as:
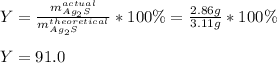
Hence, answer is D. 91.0%.
Best regards.