Answer:
Balanced reaction:
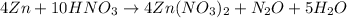
The mole ratio (4 moles Zn/5 moles
 ) is correct.
) is correct.
Step-by-step explanation:
Oxidation:
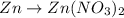
Balance N and O:
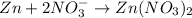
Balance charge:
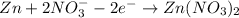 ................(1)
................(1)
Reduction:
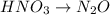
Balance N:
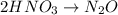
Balance H and O:
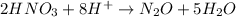
Balance charge:
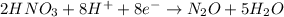 ............. (2)
............. (2)
[
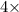 Equation-(1)] + [Equation-(2)]:
Equation-(1)] + [Equation-(2)]:
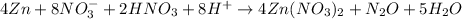
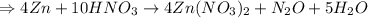
So, balanced reaction:
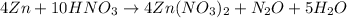
Hence the mole ratio (4 moles Zn/5 moles
 ) is correct.
) is correct.