Answer:
The missing reactant is
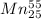 55/25Mn
55/25Mn
Step-by-step explanation:
asumming the missing reagent is A/Z X
In nuclear reaction , the sum of atomic number and mass number of on both sides musst be equal
Thus ,
Z + 54 = 79 + 0
Z = 25
The element with atomic number 25 is Mn
A + 143 = 197 +1
A = 55
Therefore, the missing reagent is 55/25Mn