Answer:
+4
Step-by-step explanation:
Hello,
In this case, considering nitrogen dioxide's formula:

And due to the fact that oxygen's oxidation state is -2 in this case, we could find nitrogen's oxidation state in nitrogen dioxide by using a balance charge:
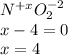
For that reason, nitrogen's oxidation state is +4:
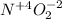
Best regards.