Step-by-step explanation:
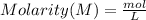
First, we need to convert 50.0 mL to L.
1 mL = 0.001 L
50.0 mL = 0.05 L
Now, we need to find the number of moles of CuSO4 when given 10.0 grams of the compound.
159.609 grams CuSO4 = 1 mol CuSO4
10.0 grams CuSO4 = 0.0627 mol CuSO4
Now, we can plug in this information into the formula.
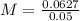
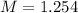
So, the molarity of copper (II) sulfate is 1.254 when given a 50.0 mL aqueous solution.