Answer:
The H+ (aq) concentration of the resulting solution is 4.1 mol/dm³
(Option C)
Step-by-step explanation:
Given;
concentration of HA,
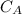 = 6.0mol/dm³
= 6.0mol/dm³
volume of HA,
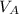 = 25.0cm³, = 0.025dm³
= 25.0cm³, = 0.025dm³
Concentration of HB,
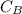 = 3.0mol/dm³
= 3.0mol/dm³
volume of HB,
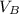 = 45.0cm³ = 0.045dm³
= 45.0cm³ = 0.045dm³
To determine the H+ (aq) concentration in mol/dm³ in the resulting solution, we apply concentration formula;
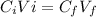
where;
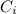 is initial concentration
is initial concentration
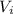 is initial volume
is initial volume
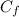 is final concentration of the solution
is final concentration of the solution
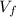 is final volume of the solution
is final volume of the solution
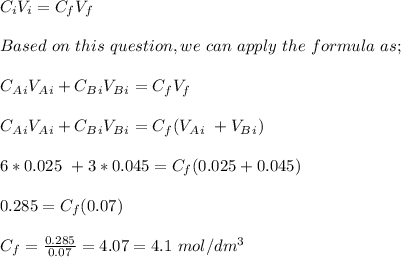
Therefore, the H+ (aq) concentration of the resulting solution is 4.1 mol/dm³