Answer:
Option D is correct
Step-by-step explanation:
The individual mass of reactants in the reaction are
Calcium Oxide (CaO) =
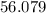 amu
amu
Carbon dioxide (CO2)
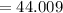 amu
amu
Since this reaction is a simple addition reaction in which no mass of the reactants is wasted. Also, no energy is released accompanied by conversion of mass into energy.
Hence, total mass of reactants would be equal to the mass of the product.
Thus, mass of calcium carbonate i.e CaCO3 is
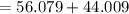
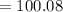 amu
amu
Hence, option D is correct