Answer:
.122 mols of KCl
Step-by-step explanation:
Read the explanation while looking at the picture.
We star with 15g because it's wat they give us:
15g of KCIO3 will cancel out with 123g of KCIO3 (molar mass)
~Grams of KCIO3 are canceled out.~
That 123g of KCIO3 is the same as 1 mol of KCIO3
Then we use mole to mole ratio:
1 mol of KCIO3 equals 2 mols of KCIO3 -- 2 moles from the equation
The 2 mols of KCIO3 is equal to 2 mols of KCl (from equation)
Multiply the numbers on top. Multiply the numbers on the bottom. Then divide. You get:
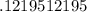
This sounds confusing. But the picture below is the format.
The SAME COLOR are the ones that cancel out.