Answer:
The new volume is 2580 mL.
Step-by-step explanation:
Charles law gives the relationship between volume and temperature of the gas. According to this law, volume of gas is directly proportional to temperature, pressure remains constant.
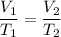
Here,
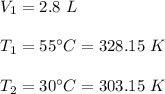
We need to find V₂. So,
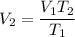
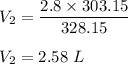
2.58 L = 2580 mL
So, the new volume is 2580 mL.