Answer:
The number of moles of oxygen present in the crew cabin at any given time is 615.309 moles
Step-by-step explanation:
The given parameters are;
Volume of the crew cabin = 74,000 L
Pressure of the crew cabin = 1.00 atm
Percentage of nitrogen in the mixture of gases in the cabin = 80%
Percentage of oxygen in the mixture of gases in the cabin = 20%
Temperature of the cabin = 20°C = 293.15 K
Therefore, volume of oxygen in the crew cabin = 20% of 74,000 L
Hence, volume of oxygen in the crew cabin =
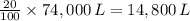
From the universal gas equation, we have;
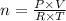
Where:
n = Number of moles of oxygen
P = Pressure = 1.00 atm
V = Volume of oxygen = 14,800 L
T = Temperature = 293.15 K
R = Universal Gas Constant = 0.08205 L·atm/(mol·K)
Plugging in the values, we have;
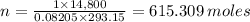
The number of moles of oxygen present in the crew cabin at any given time = 615.309 moles.