Answer:
The new pressure is 3850 torr.
Step-by-step explanation:
The relation between volume and pressure is inverse as per Boyle's law. Its mathematical form is given by :

Here,
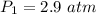
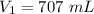
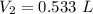
Let
 is the new pressure. So using Boyle's law we get :
is the new pressure. So using Boyle's law we get :
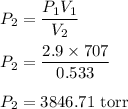
or
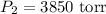
So, the new pressure is 3850 torr.