Answer:
The correct answer is 399.8 ppm
Step-by-step explanation:
A concentration in parts per million (ppm) is equal to:
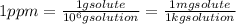
Solute: Cd; Mass = 180 mg x (1 g/1000 mg) = 0.18 g
Solvent: Water ; Mass= 450.0 g x (1 kg/1000 g) = 0.45 kg
We have the following total mass of solution:
Mass of solution = Mass of solute + Mass of solvent = 0.18 g + 450 g = 450.18 g = 0.45018 kg
Finally, we divide the mass of solute (in mg) into the mass of solution (in kg) to obtain the ppm (in mg/Kg):
ppm = 180 mg/0.45018 kg = 399.8 mg/Kg = 399.8 ppm